QuikRead go Plus Instrument
QuikRead go Plus Instrument is a practical, fully automatic instrument for analysing patient samples with QuikRead go reagent kits. QuikRead go system - consisting of the instrument and ready-to-use kits - is especially designed to be used in primary healthcare and point-of-care (POC) settings. The instrument can be connected to hospital or laboratory information systems.

Generally
QuikRead go Plus Instrument for point-of-care testing (POCT)
Accurate diagnoses and proper patient management require reliable test results. Modern healthcare settings require more than just test results; they need practical solutions. Testing needs to be fast and effortless so that treating patients is in the forefront and not managing the testing instrumentation.
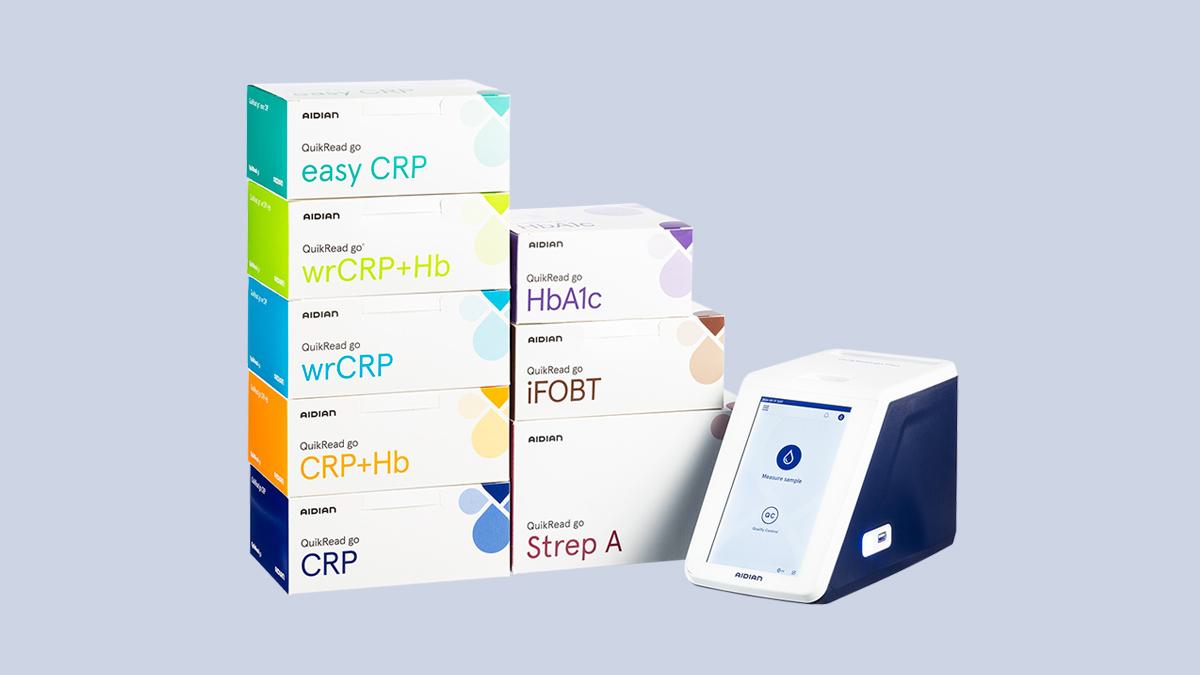
QuikRead go Plus Instrument’s intuitive user interface combined with fast results, reliability, and connectivity options make the instrument a valuable daily diagnostic tool for healthcare professionals. The reliability of the results is guaranteed by their comparability with laboratory method results1. The patient test results are fully traceable and their integrity is ensured with robust quality control management features and state-of-the-art cyber security measures.

For users looking for testing on the go, QuikRead go Plus Instrument is your lab on the move. With an ergonomic handle, long lasting battery, and other accessories you are supported for patient visits all day.
Accessories such as a barcode reader and a printer can be connected to the instrument for an even smoother workday.
Benefits of the instrument:
Intuitive measurement flow with info guides
Patient result protection
- Extensive quality control features
- State-of-the-art cyber security
Lab on the move
- Ergonomic carrying handle
- Long-lasting battery
- Small footprint
Ready-to-use reagents
- Minimal hands-on time
- Results in minutes
Effortless
- Maintenance-free
- Calibration-free
- Instrument self-checks
Principle of the instrument
QuikRead go Plus Instrument is a photometer and a turbidimeter for analysis of QuikRead go reagent kits. It measures the reaction absorbance in a cuvette and converts the value based on the test calibration data encoded on each cuvette label into a quantitative or qualitative result.
The instrument has a pre-programmed microprocessor which controls assay steps and data processing. Test identification, timing, and calibration curve or cut-off value data are stored in the barcode of each test cuvette. Once the cuvette label has activated the microprocessor, it controls and guides all assay steps and converts the sample absorbance values into concentration units or cut-off values.

User interface
The instrument has a mobile-like user interface including info icons, guiding notes, and animations to guide the user. The user interface is available in 24 languages. With the instrument's intuitive user interface, you can customize the instrument, test, quality control, and connection settings to suit your needs.
Connectivity
The various connectivity solutions of QuikRead go Plus Instrument enable an easier and safer measurement process. The instrument can be connected to LIS/HIS systems with a LAN cable or through Wi-Fi.
The instrument is compliant with LIS01-A2 and POCT1-A2 protocols.
This version of QuikRead go Plus Instrument is not registered in the USA.
References
- Performance flyer: Proven performance with QuikRead go Plus Instrument
Technical data
| Products available | 155378 QuikRead go Plus Instrument, 1 pc |
| Use | For in vitro diagnostic use, IVDR CE |
| Method | Immunoturbidimetric and photometric |
| Sample type | Analyte dependent |
| Measurement time | Analyte dependent: 2 to 6 minutes |
| Reading of the result | Instrument reads and displays the result |
| Storage | 2-35 °C |
| Size and weight | 269 (l) x 139 (w) x 166 (h) mm, weight 2.0 kg without power supply |
| Additionally needed | Test kits Optional: Rechargeable battery (Cat. no. 155231) |
| Country of origin | Finland |
| Registration | This version of QuikRead go Instrument is not registered in the USA |
| Registered trademark | QuikRead go is a registered trademark of Aidian Oy |
Photometer
The photometer consists of a measurement well, three LEDs, and a light detector. The photometer has been designed and calibrated for both photometric and turbidimetric measurements.
Memory
QuikRead go Plus Instrument has an internal memory for result history, separately for patient samples and for quality control samples. The memory capacity is 6,000 patient results, and 6,000 QC results. The results are shown on the display, can be printed, or transferred to a USB stick as .CSV file.
Printers for QuikRead go Plus Instrument
The following printers are compatible with QuikRead go Instrument:
GoDEX DT4x Label printer (Cat. no. 155129)
HP LaserJet (supported languages: PCL5 and PCL6)
HP Colour LaserJet (supported languages: PCL5 and PCL6)
Barcode reader for QuikRead go Plus Instrument
A barcode reader can be used for easy patient ID and/or operator ID reading. Aidian offers a laser barcode reader Opticon OPI-3601 (cat. no. 155389). Connect the barcode reader to one of the USB ports. Admin needs to configure the barcode reader to the same language as is used in QuikRead go Plus Instrument.
External keyboard
Any standard USB keyboard can be connected to QuikRead go Instrument.
Power supply
The power supply is supplied with the instrument. In addition, the instrument can use a battery as a power source. Rechargeable Lithium-ion battery is sold separately (Cat. no. 155231).
Requirements
- 100 - 240 V AC
- 50 - 60 Hz power supply or battery unit.
- Power consumption
- Idle 4 W
- Stand-by 1 W
- Max 35 W (without external accessories)
Connectivity
Connectivity solutions of QuikRead go Plus Instrument
Connected near-patient testing devices transfer measurement results automatically to a data handling system, from where they are directly distributed to laboratory (LIS) and hospital information systems (HIS). By reducing the possibility of mistyping in recording the results, automatic data transfer increases patient safety as well as speeds up patient treatment.
Connectivity improves the traceability of test results when patient and operator identifiers are added to the measurement data.
QuikRead go Plus Instrument can be connected to LIS using either a uni-directional or bi-directional connection.
Uni-directional connection
QuikRead go Plus Instrument can be connected to any systems that are capable of communicating with QuikRead go Plus Instrument using the LIS01-A2 (ASTM) standard. For this purpose, the system requires an LIS01-A2 driver for QuikRead go Plus Instrument.
After a test measurement has been performed, QuikRead go Plus Instrument automatically sends a LIS01-A2 message with the following information:
- Patient ID (if patient ID is on)
- Measurement serial number
- Analyte information / Test ID
- Result and result unit
- Operator ID (if operator ID is on)
- Measurement time and date
- System name and serial number information
Bi-directional connection
QuikRead go Plus Instrument can be connected to systems that are capable of communicating with QuikRead go Plus Instrument using the POCT1-A2 standard. For this purpose, the system requires a POCT1-A2 driver for QuikRead go Plus Instrument.
After a test measurement has been performed, QuikRead go Plus Instrument automatically sends a POCT1-A2 message with the following information:
- Patient ID
- Measurement serial number
- Analyte information / Test ID
- Result and result unit
- Operator ID (if operator ID is on)
- Measurement time and date
- System name and serial number information
The features of the bi-directional connection enable users to:
- Download patient lists
- Manage operator lists
- Lock/unlock instruments
- Tag patient/QC results with comments
- Send messages to operators
- Send instrument error info, instrument notifications
- Set QC limits
- Set the time
- Change settings
- Update software
Connection types
LIS01-A2 or POCT1-A2 connection using TCP/IP LAN connection via RJ-45
An RJ-45 connector can be used for direct network connection to LIS/HIS connections with a standardized cable.
LIS01-A2 or POCT1-A2 connection using TCP/IP WLAN connection
QuikRead go Plus software update
The instrument is ready for use straight out from the delivery box. No software installation is required prior to use. Software updates are available at softwareupdate.quikread.com, or they can be ordered on a USB stick. (Cat. no. 155433 Software USB stick for QuikRead go Plus)
Instructions for use
QuikRead go Plus Software-Update IFU (GB, FR, DE, NL, CZ, SK, HU, PL, SE, NO, DK, FI)
QuikRead go Plus Instrument software 2.3.2 release note (EN)
Frequently asked questions
What type of regular maintenance does QuikRead go Plus Instrument require?
The instrument doesn’t need any regular maintenance. The instrument is factory calibrated and has a self-check routine on startup and before every measurement to ensure functionality. The calibration curve or cut-off value for each test is encoded on the cuvette label.
How can I clean the instrument?
Wipe the exterior with a damp lint-free cloth. A mild detergent may be used if necessary. Avoid spills and don’t let water enter the instrument through the measurement well or the connectors. Do not use organic solvents or corrosive substances. Potentially infectious material should be wiped off immediately with absorbent paper tissue and the contaminated areas swabbed with 70% ethyl alcohol, Desicton (Kiilto), 0.5 % sodium hypochlorite or Super Sani-Cloth® Germicidal Disposable Wipe. Materials used to clean spills, including gloves, should be disposed of as biohazardous waste.
Does the software need updating?
The instrument is ready to use straight out from the delivery box and no software updates are needed prior starting to use the instrument. Software updates are made available when new features are available, such as:
- new analytes and reagent kits
- optional ways to connect the instrument to LIS systems
- extended result memory
- improvements to existing functionalities i.e. bug fixes
If you would like to access the new features, software updates are necessary.
How are software updates delivered?
You can download the latest software version from www.softwareupdate.quikread.com. Alternatively you can order the update file on a USB storage from your local distributor. The software is easy to update using the instrument’s user interface as follows: Menu => Maintenance => Software update. Follow the instructions on the display.
Is QuikRead go Plus Instrument connectable to my lab computer software?
Yes, if it has a driver for QuikRead go Plus Instrument. The connection can be uni-directional or bi-directional. A uni-directional connection is based on the LIS01-A2 standard. Here the information flow is from QuikRead go Instrument to the LIS/HIS/Middleware. A bi-directional connection is based on the POCT1-A2 standard. Here information goes both ways, from the instrument to the network (LIS/HIS/MW) and vice versa allowing remote management of QuikRead go Plus instruments and the users.
What is a driver?
A driver is software that tells the operating system (LIS/HIS/MW) and other software how to communicate with the connected device. For example, all printers come accompanied with drivers to be installed and that enable printing.
What are LIS01-A2 and POCT1-A2 protocols?
The LIS01-A2 and POCT1-A2 protocols are standardized ways for transferring information between laboratory instruments and computer systems.
What is Middleware?
Middleware is a software system that enables communication between a test device and a laboratory or hospital data handling system. There are Middleware systems which have been especially designed to manage several POC-devices and users simultaneously.
GMT time
If you inadvertently set GMT to a future time, it can be readjusted back to the correct time IF the instrument has not been shut down in between. If the instrument has been shut down, setting GMT backwards is not possible but the instrument has to be sent to Finland for service. Alternatively, you can
1. Disconnect the power supply.
2. Remove rechargeable battery, if that has been placed into the instrument.
3. Remove the clock battery, and then wait until you reach the erroneously set date.
How many measurements can you perform with a fully charged battery?
You can measure approximately 100 measurements with a fully charged battery.
How can I charge the battery?
The battery charges when the power cable of the instrument is connected even if the instrument is turned off.
How do I know when charging is needed?
The charge of the battery is indicated in the status bar when the instrument is battery powered and turned on.
How long does it take to fully charge the battery?
Full charging time is approximately 5 hours.
Recommended settings
To save the battery charge, set the sleep mode to “Full stand-by” and set the delay to the lowest value. You can change the settings from Menu > Settings > Power saving
How should QuikRead go Plus Instrument be disposed of?
QuikRead go Plus Instrument is a low-voltage electronic device. A used QuikRead go Plus Instrument must be treated as potentially infectious waste. The instrument should be disposed of as electrical and electronic equipment (WEEE 2012/19/EU) if local and national laws do not require the instrument to be collected and disposed of as potentially infectious clinical waste.
The packaging materials are recyclable.
Be sure to disinfect the device and to delete all patient data before disposing of the instrument.
Read more: Disposal of QuikRead go Plus Instrument
Disposal of QuikRead go Plus Instrument
QuikRead go Plus Instrument is a low-voltage electronic device. A used QuikRead go Plus Instrument must be treated as potentially infectious waste. The instrument should be disposed of as electrical and electronic equipment (WEEE 2012/19/EU) if local and national laws do not require the instrument to be collected and disposed of as potentially infectious clinical waste.
The packaging materials are recyclable.
Be sure to disinfect the device and to delete all patient data before disposing of the instrument.
Disinfection
Before sending QuikRead go Plus Instrument to recycling clean the external surfaces and decontaminate the interior of the instrument.
Clean the exterior of the instrument using a lint-free cloth dampened with water. If necessary, a mild detergent may be used. Do not use organic solvents or corrosive substances. Spillage of potentially infectious material should be wiped off immediately with absorbent paper tissue and the contaminated areas swabbed with 70% ethyl alcohol or standard disinfectant. For safety, use chemical resistant gloves and follow the instructions in the safety data sheet. Materials used to clean spills, including gloves, should be disposed of as potentially infectious waste.
To decontaminate the inside of the device, open the lid and spray 5% sodium hypochlorite into the instrument through the cuvette hole or if not able to open lid, access interior of the instrument by removing the accumulator unit cover at the bottom of the instrument and opening the bottom hatch.
Do not spray disinfectant on the accumulator of battery.
Let the disinfectant dry for 24 h and in addition, the device must be aged for two weeks after the last use the device before sending QuikRead go Plus Instrument for recycling. For your safety, use chemical-resistant gloves, and follow the instructions in the safety data sheet of the disinfectant.
Data protection
Used QuikRead go Plus Instrument may contain sensitive patient data. Please note, that the end user of the device is responsible for deleting all the data on the old devices to be disposed of. To delete the data, log in as Admin and go to Menu > Maintenance > Patient results > Delete patient result list.
QuikRead go Plus Instrument contains the SVHC substances that are bound inside the device and not released during regular use. Waste operators can utilize the SCIP-database to get more information. For the SVHC substances, see QuikRead go Plus Instrument’s REACH/SVHC Statement.
QuikRead go Plus Instrument’s REACH/SVHC Statement
Country-specific instructions for recycling:
If your country's recycling instructions are not found in the list above, please contact your local distributor or Aidian office.
Documents and materials
Marketing and sales materials
QuikRead go Plus Family Brochure (EN)
QuikRead go Plus Technical Specifications (EN)
QuikRead go Plus Technical Specifications short (EN)
QuikRead go Plus Connectivity Sales Sheet (EN)
Instruction for use
(For informative use only. Kindly always refer to the latest package insert in the kit.)
QuikRead go Plus Instrument IFU (EN, DE, FR, NL) 155378, 155377
QuikRead go Plus Instrument IFU (CZ, SK, HU, PL) 155375
QuikRead go Plus Instrument IFU (FI, SE, NO, DK) 155375
QuikRead go Plus Instrument IFU (ES, PT, IT, EE, RO) 155378
QuikRead go Plus Instrument IFU (SI, RS, HR, GR) 155378
Revision history QuikRead go Plus Instrument IFU (EN, DE, FR, NL) 155378, 155377
Revision history QuikRead go Plus Instrument IFU (CZ, SK, HU, PL) 155375
Revision history QuikRead go Plus Instrument IFU (FI, SE, NO, DK) 155375
Revision history QuikRead go Plus Instrument IFU (ES, PT, IT, EE, RO) 155378
Revision history QuikRead go Plus Instrument IFU (SI, RS, HR, GR) 155378


