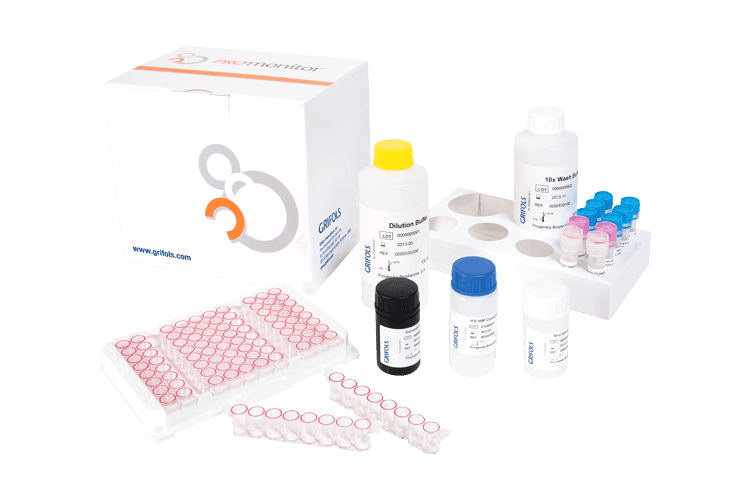
Grifols Promonitor® ELISA
Promonitor® ELISA tests are used to monitor the effectivity of biological drug treatments.
Yleisesti
The Promonitor® family of tests measures both drug levels and anti-drug antibody levels with validated ELISA assays to complement biological therapies for drug monitoring. Promonitor® kits can quantify not only biological drug levels, but also biosimilar drugs. Promonitor® tests can be performed manually or fully automated in any ELISA open processor.
The following tests are available for testing of drug bioavailability and immunogenicity:
- Promonitor® IFX and ANTI-IFX (Infliximab and anti-Infliximab antibodies)
- Promonitor® ADL and ANTI-ADL (Adalimumab anti- Adalimumab antibodies)
- Promonitor® ETN and ANTI-ETN (Etanercept anti- Etanercept antibodies)
- Promonitor® RTX and ANTI-RTX (Rituximab anti- Rituximab antibodies)
- Promonitor® GLM and ANTI-GLM (Golimumab anti- Golimumab antibodies)
- Promonitor® VDZ and ANTI-VDZ (Vedolizumab anti- Vedolizumab antibodies)
- Promonitor® UTK and ANTI-UTK (Ustekinumab anti- Ustekinumab antibodies)
Promonitor® ELISA tests are easy to use
- Multiple configurations
- Removable strips
- Microplate precoated
- Ready-to-use reagents
- Quick procedure with only 30 minutes of hands-on-time
Promonitor® ELISA tests provide you with
- Early information about reduced drug efficacy
- Individualized treatment for every patient
- Effective management of resources
Tekniset tiedot
| Products available |
|
| Use | For in vitro diagnostic use |
| Method | ELISA assay |
| Sample type | Serum |
| Time to result | 2-3 hours |
| Expiration date | 24 months |
| Storage | 2-8°C |
| Registered trademark | The Promonitor® brand is registered in several countries, including Finland, by Progenika Biopharma S.A. |
Markkinointimateriaalit
Lisätietoja
More information about Promonitor® tests is available at: Promonitor Kits for ELISA for Therapeutic Drug Monitoring | Grifols


