Benefits of Bladder EpiCheck for bladder cancer surveillance
As November, Bladder Health Month 2023, is coming to an end we want to highlight Bladder Cancer surveillance.
Bladder cancer is the 5th most common cancer in Europe, affecting over 200 000 people yearly.1 Non-muscle-invasive bladder cancer (NMIBC) accounts for the majority of newly diagnosed bladder cancers. With up to 70% of patients experiencing recurrence within 2 years of initial treatment and 10-15% progressing to higher risk cancer, monitoring of bladder cancer is vital.2
The current gold standard methods for bladder cancer surveillance are cystoscopy and cytology. However, they have their limitations as both are based on operator-dependent assessment. Cytology has low to moderate sensitivity, while some types of tumours are easily missed in cystoscopy.2
During the first years after initial treatment, bladder cancer patients undergo as many as 4 cystoscopies a year. Cystoscopy is an invasive and expensive procedure, so the need for an effective biomarker test for surveillance is high.
Bladder EpiCheck® from Nucleix is a urine marker test for monitoring of NMIBC. It uses a panel of 15 DNA methylation markers and is included in the EAU (European Association of Urology) guidelines for bladder cancer surveillance. The guidelines state that Bladder EpiCheck could be used to replace and/or postpone cystoscopy.3
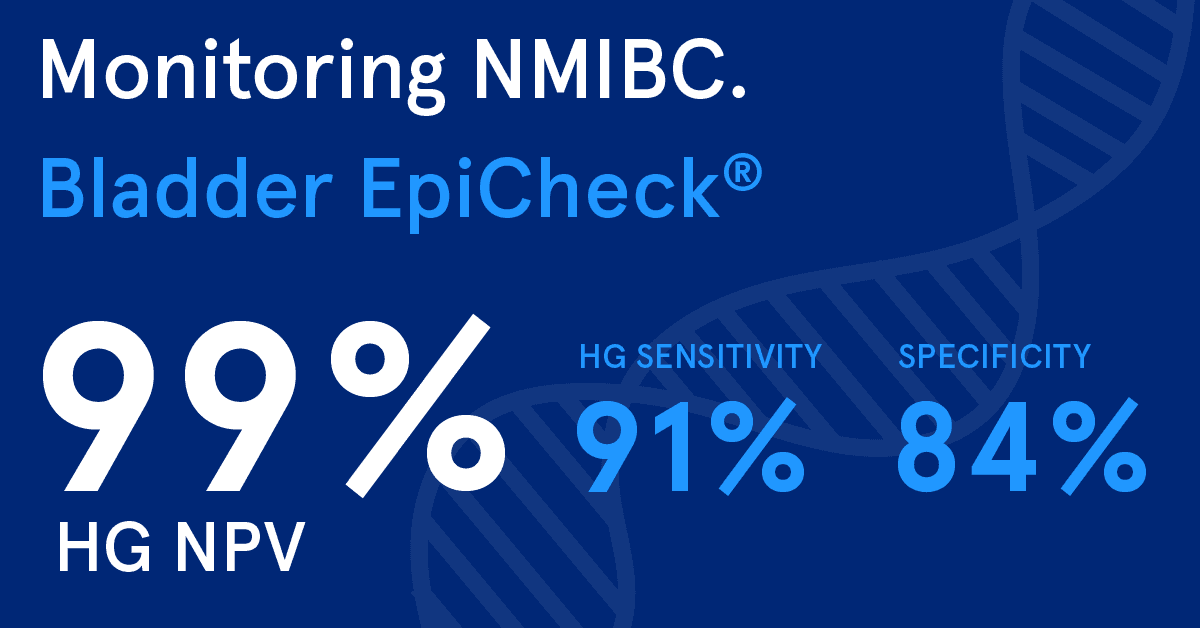
A meta-analysis of the diagnostic accuracy of urinary biomarker tests found that Bladder EpiCheck has a high-grade Negative Predictive Value (NPV) of 99 %, high-grade sensitivity of 91 % and specificity of 84 %.4
The results of the test are objective and not affected by BCG treatment, hematuria, or infections.5 The high performance of the test makes it a reliable tool to rule out high-risk recurrence and reduce unnecessary procedures.
Reducing the number of cystoscopies by incorporating Bladder EpiCheck into the surveillance of bladder cancer could lessen the strain on the healthcare system as well as benefit the well-being of patients.
For more detailed information about Bladder EpiCheck, head to the Bladder EpiCheck product site or contact us.
Aidian is the proud distributor of Bladder EpiCheck in the Nordics.
References
- European Association of Urology. Bladder Cancer: The Forgotten Cancer. https://uroweb.org/news/bladder-cancer-the-forgotten-cancer. Accessed 27.11.2023.
- Biomarkers in the management of non-muscle invasive bladder cancer (NMIBC), BCaMonitor, Updated May 2023.
- EAU Non-muscle-invasive Bladder Cancer guidelines 2022. https://uroweb.org/guidelines/non-muscle-invasive-bladder-cancer/chapter/diagnosis
- Laukhtina et al. Diagnostic Accuracy of Novel Urinary Biomarker Tests in Non-muscle-invasive Bladder Cancer: A Systematic Review and Network Meta-analysis. Eur Urol Oncol 2021 Dec;4(6):927-942; and corrigendum at Eur Urol Oncol. 2022 Jan 19;S2588-9311(22)00004-9.
- Cochetti et al. Urol Onc Nov 2021; Pierconti et al. Urol Onc Nov 2021, Bladder EpiCheck® EU Instructions For Use, 09-May-2022


